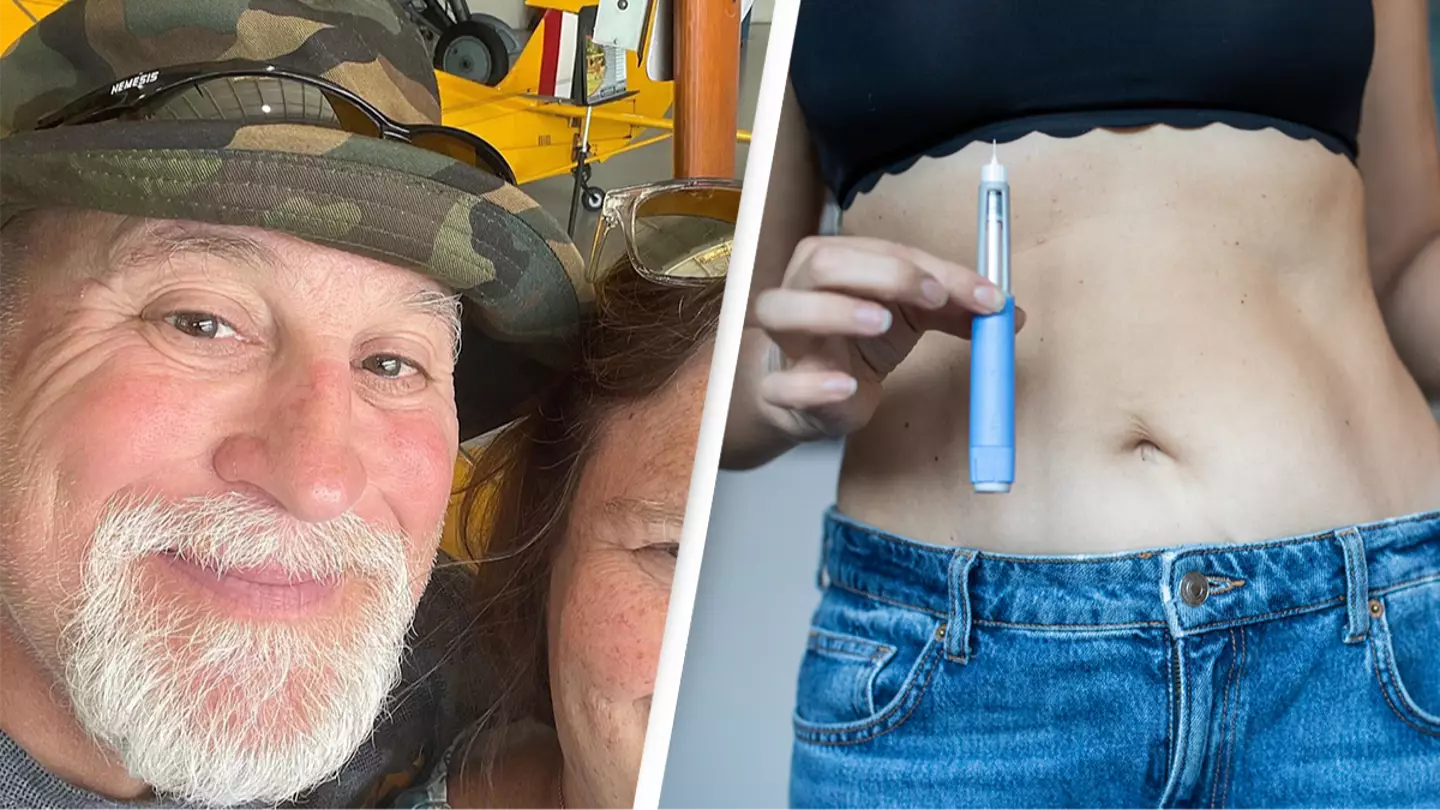
A man is suing the manufacturers of Ozempic, alleging the diabetes drug caused him to go blind.
The medication, containing semaglutide, works by regulating blood sugar and suppressing hunger.
While only FDA-approved to treat diabetes, it's boomed in popularity for weight loss purposes.
Every medication comes with side effects, but now a Maryland man is warning Ozempic users of a potential risk he says manufacturers Novo Nordisk are failing to inform patients of.
Todd Engel was prescribed the GLP-1 agonist medication in 2023 to manage his type 2 diabetes.
Ozempic has boomed in popularity (Iuliia Burmistrova/Getty Images) While on the medication, Engel's attorney, Jonathan Orent, of plaintiff litigation firm Motley Rice, said Engel first experienced loss of vision in one eye before going on to become legally blind in both.
The way the 62-year-old interacts with his family has been 'forever changed,' while Engel was also forced to resign from his job and can no longer drive, Orent said.
After four months of use, Engel was diagnosed with nonarteritic anterior ischemic optic neuropathy (NAION), the lawsuit claims.
This is a condition that causes 'sudden, painless vision loss in one eye due to a lack of blood flow to the optic nerve,' Brigham and Women's Hospital explains.
Around 6,000 new cases of NAION are diagnosed in the US each year.
While diabetes itself is a known risk factor, recent studies suggest that semaglutide - the active ingredient in Ozempic - may further elevate that risk.
NAION affects people aged 66 on average (Phil Fisk/Getty Images) A 2024 study found that people with diabetes taking semaglutide were over four times more likely to develop NAION than those who were not.
The lawsuit, filed on April 24 in the Superior Court of New Jersey, accuses Novo Nordisk of negligence and claims the company failed in its duty to inform patients and doctors about the potential risk of severe eye conditions, reports NBC News.
According to the filing, Novo Nordisk had access to data from early Ozempic clinical trials that documented cases of NAION, but opted not to include the condition on the drug’s warning label.
Orent said had Engel known about a possible link to blindness, he would have considered other treatment options.
Engel is taking Novo Nordisk to court after losing his sight (Victor Golmer/Getty Images) A Novo Nordisk spokesperson told UNILAD: "NAION is a very rare eye disease, and it is not a recognised adverse drug reaction for the marketed formulations of semaglutide (Ozempic, Rybelsus and Wegovy) as per the approved labels.
"After a thorough evaluation of studies from the University of Southern Denmark and Novo Nordisk’s internal safety assessment, Novo Nordisk is of the opinion that the benefit-risk profile of semaglutide remains unchanged.
"Novo Nordisk, on its part, has conducted an analysis across randomized controlled clinical trials with GLP-1 receptor agonists, including a blinded ophthalmologist evaluation to confirm NAION diagnoses.
"These data do not suggest a causal relationship between GLP-1 RA use and NAION events."
They continued: "Patient safety is a top priority for Novo Nordisk, and we take all reports about adverse events from use of our medicines very seriously.
"This also relates to eye conditions, which are well-known comorbidities for people living with diabetes. Any decision to start treatment with prescription-only medicines should be made in consultation with a healthcare professional who should do a benefit-risk evaluation for the patient in question, weighing up the benefits of treatment with the potential risks."
 Ellie Kemp
Ellie Kemp