A drug manufacturer has revealed how much weight can be lost on their daily GLP-1 pill.
Pharma giant Eli Lilly is currently trialing Orforglipron, a GLP-1 receptor agonist that looks set to rival Ozempic and similar weight loss medication.
While the likes of Ozempic and Mounjaro are only approved to treat diabetes, other injectables, such as Wegovy, are prescribed purely for weight loss.
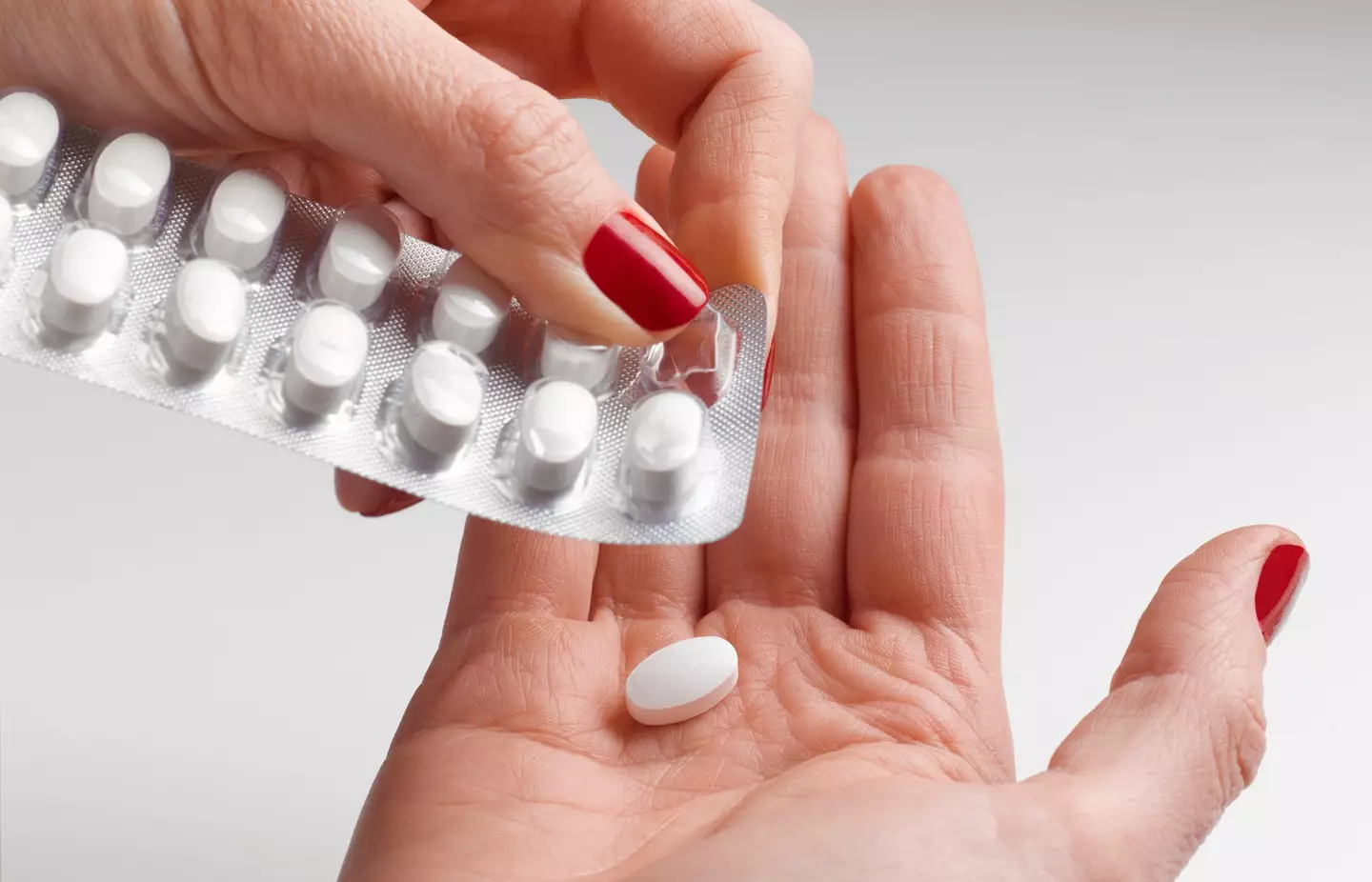
Soon, injections could become a thing of the past, with a daily pill yielding incredible results.
Advert
The tablet, taken orally once a day, works by regulating both blood sugar and appetite, leading to weight loss.
Lilly hopes to have its Orforglipron pill signed off by the end of the year - with the tablet not only helping participants lose a 'significant' amount of weight, but majorly improving heart health, too.
It comes as the US-based pharmaceutical company reported $15.56 billion in revenue for its second quarter on Thursday (August 7), putting its earning per share at $6.31, beating Wall Street's estimates of $5.56.
What did the Orforglipron study find?
More than 3,000 adults who were either obese or overweight with health problems - but didn't have diabetes - took part in the pill's phase three trials.
The goal was to see how well Orforglipron helped with weight loss when combined with a healthy diet and exercise.
People taking Orforglipron once a day lost significantly more weight than those who took a placebo.
By taking 36 mg once per day - without food and water restrictions - participants lost an average of 12.4 percent of their body weight - about 27.3 pounds (12.4 kilograms). This was over the span of 72 weeks - a little more than 16 months.
This compares to just 0.9 percent - 2.2 pounds - for people on the placebo.
The study found almost 60 percent of people on the highest dose lost at least 10 percent of their body weight.
Almost 40 percent lost 15 percent or more of their body weight. And that's not all...
How did Orforglipron impact heart health?

Participants not only achieved substantial weight loss, but the pill also showed positive effects on heart health.
After 72 weeks, individuals had reduced levels of harmful cholesterol, lower triglycerides - a type of blood fat linked to higher risk of heart attack and stroke -and improved blood pressure.
What did previous Orforglipron trials show?
In a previous, 40-week trial, Orforglipron was found to have lowered peoples' A1C - a key blood sugar marker - by up to 1.6 percent.
Two-thirds of people on the highest dose, 36 milligrams, ended up with blood sugar levels below the diabetes threshold.
Researchers also found participants who took the highest dose lost an average of eight percent of their body weight - around 16 pounds.
What happens next?

Eli Lilly plans to submit the drug for regulatory approval by the end of the year, aiming for a global launch.
'Substantial investments' are also being made to meet anticipated demand at launch.
Kenneth Custer, Ph.D., executive vice president and president of Lilly Cardiometabolic Health, confirmed: "Obesity is one of the most pressing global health challenges of our time, driving global chronic disease burden and impacting more than one billion people worldwide.
"With Orforglipron, we're working to transform obesity care by introducing a potential once-daily oral therapy that could support early intervention and long-term disease management, while offering a convenient alternative to injectable treatments.
"With these positive data in hand, we are now planning to submit orforglipron for regulatory review by year-end and are prepared for a global launch to address this urgent public health need."